Intro
Discover the molar weight of air and its significance in various fields. Learn how to calculate the molecular weight of air, its composition, and the importance of average molar mass in physics, chemistry, and engineering applications, including atmospheric science and gas dynamics.
Air is a mixture of gases that surrounds our planet, and its composition is not a fixed value. The molar weight of air, also known as the molar mass of air, is a concept that helps us understand the average molecular weight of the air we breathe. Understanding the molar weight of air is essential for various applications, including chemistry, physics, and engineering.
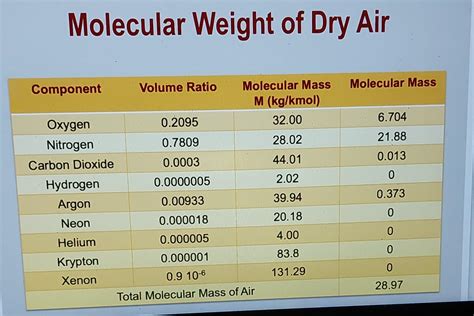
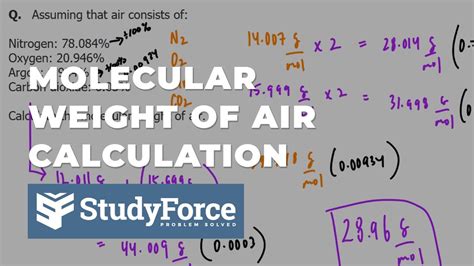
The molar weight of air is not a straightforward value, as it is a mixture of different gases. The primary components of air are nitrogen (N2), oxygen (O2), argon (Ar), and carbon dioxide (CO2), with trace amounts of other gases such as neon (Ne), helium (He), methane (CH4), and water vapor (H2O). The proportions of these gases can vary depending on factors such as location, altitude, and weather conditions.

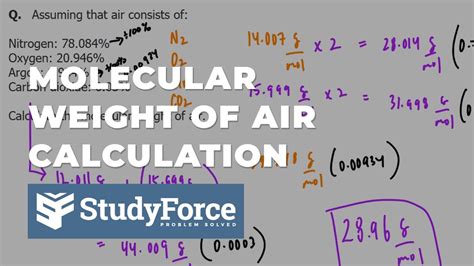
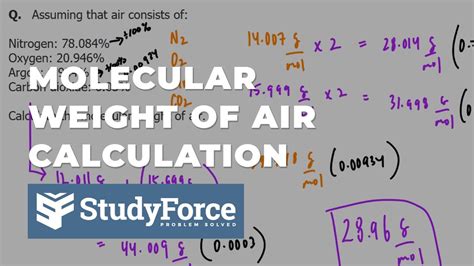
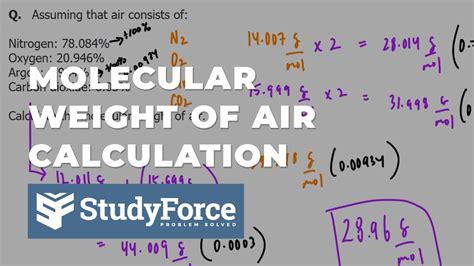
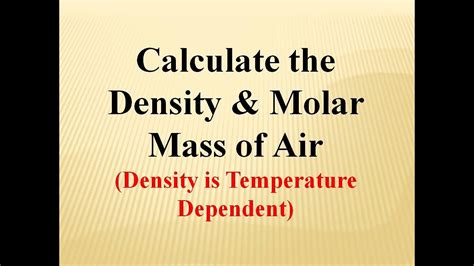
To calculate the molar weight of air, we need to consider the average molecular weight of its components. The molar mass of a gas is the sum of the atomic masses of its constituent atoms. Using the average atomic masses of the elements, we can calculate the molar mass of air as follows:
Molar mass of air ≈ (0.7808 × 28.01 g/mol) + (0.2095 × 32.00 g/mol) + (0.0093 × 39.95 g/mol) + (0.0005 × 44.01 g/mol) Molar mass of air ≈ 28.97 g/mol
The molar weight of air is approximately 28.97 grams per mole (g/mol). This value represents the average molecular weight of the air we breathe, taking into account the varying proportions of its components.
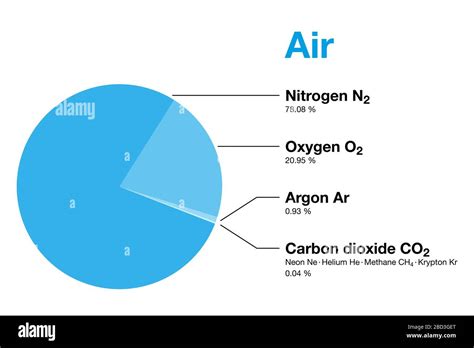
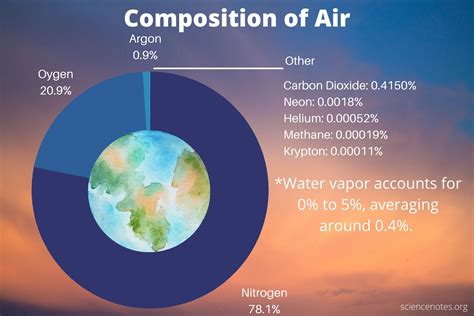
Composition of Air
To understand the molar weight of air, it is essential to know the composition of air. The major components of air are:
- Nitrogen (N2): 78.08%
- Oxygen (O2): 20.95%
- Argon (Ar): 0.93%
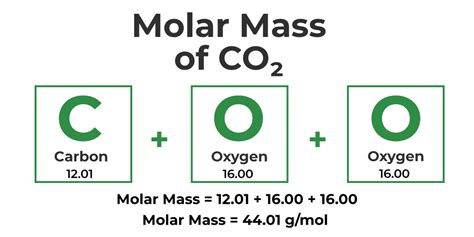
- Carbon dioxide (CO2): 0.05%
- Water vapor (H2O): variable (average 1%)
Other gases present in air include:
- Neon (Ne): 0.0018%
- Helium (He): 0.0005%
- Methane (CH4): 0.0002%
- Hydrogen (H2): 0.0001%
Factors Affecting the Molar Weight of Air
Several factors can influence the molar weight of air, including:
- Location: The molar weight of air can vary depending on the location due to differences in atmospheric pressure, temperature, and humidity.
- Altitude: As altitude increases, the air pressure decreases, which can affect the molar weight of air.
- Weather conditions: Weather conditions such as temperature, humidity, and wind can influence the molar weight of air.
- Air pollution: The presence of air pollutants can alter the molar weight of air.
Molar Weight of Air at Different Altitudes
The molar weight of air can vary with altitude due to changes in air pressure. At higher altitudes, the air pressure decreases, which can affect the molar weight of air.
| Altitude (m) | Molar Weight of Air (g/mol) |
|---|---|
| 0 (sea level) | 28.97 |
| 1000 | 28.92 |
| 2000 | 28.86 |
| 3000 | 28.79 |
| 4000 | 28.72 |
Importance of Molar Weight of Air
The molar weight of air is essential for various applications, including:
- Chemical reactions: Understanding the molar weight of air is crucial for predicting the outcomes of chemical reactions that involve air as a reactant or product.
- Engineering: The molar weight of air is important for designing and optimizing systems that involve air, such as air compressors, pneumatic systems, and air conditioning systems.
- Physics: The molar weight of air is used in calculations involving the behavior of gases, such as the ideal gas law and the kinetic theory of gases.
In conclusion, the molar weight of air is an important concept that helps us understand the average molecular weight of the air we breathe. While the molar weight of air is approximately 28.97 g/mol, it can vary depending on factors such as location, altitude, and weather conditions. Understanding the molar weight of air is essential for various applications, including chemistry, physics, and engineering.
Molar Weight Of Air Image Gallery



What is the molar weight of air?
+The molar weight of air is approximately 28.97 grams per mole (g/mol).
What factors affect the molar weight of air?
+Factors that can affect the molar weight of air include location, altitude, and weather conditions.
Why is the molar weight of air important?
+The molar weight of air is essential for various applications, including chemistry, physics, and engineering.
Share your thoughts on the importance of the molar weight of air in the comments section below. Don't forget to share this article with your friends and colleagues who might find it useful.

