Intro
Discover the 5 key properties of matter with our comprehensive anchor chart guide. Learn about the defining characteristics of matter, including mass, volume, density, buoyancy, and states of matter. Perfect for science students and teachers, this visual aid simplifies complex concepts, making it easy to understand and visualize the fundamental properties of matter.
Understanding the fundamental properties of matter is crucial in various fields of science, technology, engineering, and mathematics (STEM). Matter, which makes up everything around us, exhibits several key properties that help us distinguish one substance from another. In this article, we will delve into the five essential properties of matter and explore their significance in our daily lives.
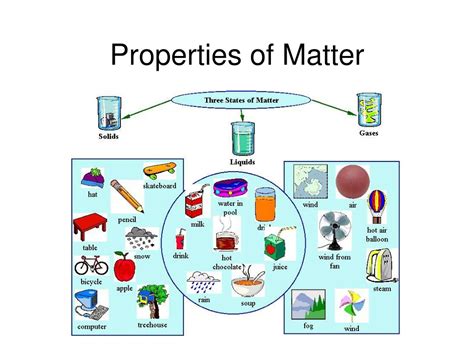
Matter's properties can be broadly classified into physical and chemical properties. Physical properties can be observed or measured without altering the substance's chemical composition, whereas chemical properties involve changes in the chemical structure of the substance. Let's examine the five key properties of matter, which are essential in understanding the behavior of substances in various environments.
1. Mass
Mass is a fundamental property of matter that represents the amount of matter present in an object or substance. It is typically measured in units of kilograms (kg) or grams (g) and is often confused with weight. However, mass and weight are distinct properties; mass remains constant regardless of the object's location, whereas weight is dependent on the gravitational force acting upon the object.

Understanding mass is crucial in various scientific applications, such as calculating the density of a substance, determining the amount of matter in a given volume, and predicting the behavior of objects in different gravitational environments.
Measuring Mass
Mass can be measured using various techniques, including:
- Balance scales: comparing the mass of an object to a known standard
- Electronic balances: using a digital scale to measure mass
- Hydrostatic weighing: measuring the buoyancy of an object in a fluid
2. Volume
Volume is another essential property of matter that represents the amount of space occupied by an object or substance. It is typically measured in units of liters (L), milliliters (mL), or cubic meters (m³). Understanding volume is crucial in various applications, such as calculating the density of a substance, determining the capacity of a container, and predicting the behavior of fluids.

Measuring Volume
Volume can be measured using various techniques, including:
- Rulers: measuring the length, width, and height of an object
- Graduated cylinders: measuring the volume of a liquid
- Displacement: measuring the volume of an object by displacing a fluid
3. Density
Density is a derived property of matter that represents the mass per unit volume of a substance. It is typically measured in units of kilograms per cubic meter (kg/m³) or grams per milliliter (g/mL). Understanding density is crucial in various applications, such as predicting the behavior of objects in different environments, calculating the buoyancy of an object, and determining the composition of a substance.

Calculating Density
Density can be calculated using the formula:
density = mass / volume
4. Melting Point
Melting point is a physical property of matter that represents the temperature at which a substance changes state from solid to liquid. It is typically measured in units of degrees Celsius (°C) or Kelvin (K). Understanding melting point is crucial in various applications, such as predicting the behavior of substances in different environments, determining the composition of a substance, and developing new materials.
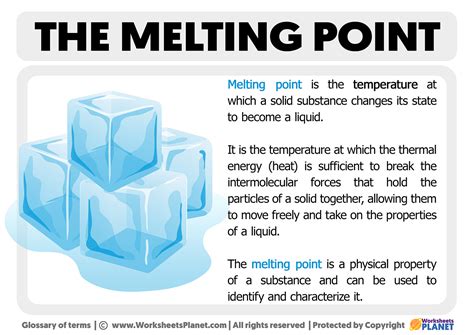
Measuring Melting Point
Melting point can be measured using various techniques, including:
- Thermocouples: measuring the temperature of a substance as it melts
- Melting point apparatus: using a specialized device to measure melting point
5. Boiling Point
Boiling point is a physical property of matter that represents the temperature at which a substance changes state from liquid to gas. It is typically measured in units of degrees Celsius (°C) or Kelvin (K). Understanding boiling point is crucial in various applications, such as predicting the behavior of substances in different environments, determining the composition of a substance, and developing new materials.

Measuring Boiling Point
Boiling point can be measured using various techniques, including:
- Thermocouples: measuring the temperature of a substance as it boils
- Boiling point apparatus: using a specialized device to measure boiling point
Properties of Matter Image Gallery






What is the difference between mass and weight?
+Mass and weight are often confused with each other, but they are distinct properties. Mass represents the amount of matter present in an object, whereas weight is dependent on the gravitational force acting upon the object.
How is density calculated?
+Density is calculated by dividing the mass of a substance by its volume. The formula for density is: density = mass / volume.
What is the significance of melting point in everyday life?
+Melting point is crucial in various applications, such as predicting the behavior of substances in different environments, determining the composition of a substance, and developing new materials.
As we have explored the five key properties of matter, it is evident that understanding these properties is essential in various scientific applications and everyday life. By recognizing the significance of mass, volume, density, melting point, and boiling point, we can better appreciate the complexities of the world around us and develop new technologies and materials that improve our lives.
We hope this article has provided you with a comprehensive understanding of the properties of matter. If you have any questions or topics you would like to discuss, please feel free to comment below.
